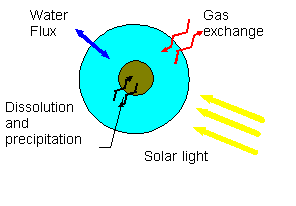
Modelling trace metals acting as catalysts in clouds:
multiphase chemistry in non polluted area.
CMD project APP 8, Karlsruhe 23-25 September 1998
R. Losno, K. Desboeufs
Laboratoire Interuniversitaire des Systèmes Atmosphériques (LISA)
Universités Paris 7 Paris 12, UMR CNRS 7583
e-mail: losno@lisa.univ-paris12.fr
Introduction
Trace metals, and especially d-block metals (Fe, Cu and Mn) are
found to be essential to describe the aqueous atmospheric chemistry.
The influence of dissolved trace metals in cloud droplets should
be compared to other factors. We will present case studies applied
to non polluted air masses, including hydrogen peroxide, ozone
and small amounts of background crustal aerosols. Two coupled
catalytic cycles drive all the reaction scheme: One with iron
and other with copper. Gas exchange is fast enough to be considered
as equilibrium, but metals exchanges between particulate and dissolved
phase are of major importance. All the concentrations are given
in mol.L1 (M) in the figures.
Cloud drop chemical sheme
The chemistry inside the cloud droplet in remote areas can be
well described by around 40 reactions [1-5]. But most of them
are not important because not fast enough. The initial concentrations
are taken following next table:
| Compound | Initial concentration | Reference |
| O3 | 3.0 E-10 mol.L1 | 25 ppb gaseous |
| H2O2 | 10 µmol.L1 | [6] |
| H+ | 10 µmol.L1 (pH = 5) | [7], [8] |
| Soluble Fe | 5.0 E8 mol.L1 | [10], [11] |
| Soluble Mn | 3.0 E9 mol.L1 | [10], [12] |
| Soluble Cu | 1.0 E-9 mol.L1 | [12], [13], [14] |
Reduced system
The system can be summarised by the following main equations, giving two catalytic cycles, one with FeIII/FeII and another with CuII/CuI:
H2O2 + hv OH + OH J1=5.70 10-07 s1
H2O2 + OH HO2 k4=2.70
10+07 M1.s1
O2- + FeIII FeII + O2 k19,20=1.50 10+08 M1.s1
H2O2 + Cu+ Cu2+ + OH k35=4.00 10+05 M1.s1
O2- + Cu2+ Cu+ + O2 k38=5.00 10+09 M1.s1
hv + Fe(OH)2+ FeII + OH J22=5.90 10-04 s1
H2O2+ Fe(OH)+ FeIII
+ OH k28=6.01 10+01 M1.s1
A photostationary state is reached when (Ka;HO2 is
the acidity constant of HO2):
Reactivity dynamics
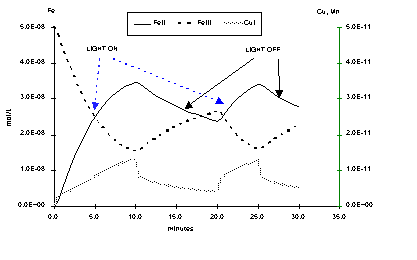
As the system reacts very fast to irradiation changes, this state is reached after less than 1 second (next figure).

But FeII/FeIII variations are slower which allows iron speciation measurements. By opposite, the copper speciation is faster relaxed because reaction constant between copper and radicals are greater than between iron and radicals. Next figure shows long term variations of CuI, FeII and FeIII concentrations in a periodically illuminated system. Right scale is for CuI, which concentration varies around 1011 mol.L1. CuII remains constant. "LIGHT ON" is a period where all the J are to their nominal values, and "LIGHT OFF" where all J are switched to zero.
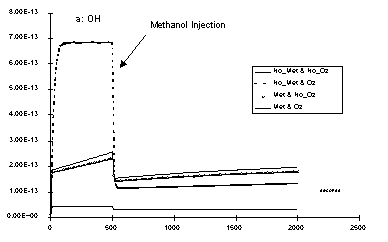
Thus, the chemical system is also more stable against chemical
variations. The following example shows the OH radical concentrations
versus time if methanol (107 mol.L1)
is added to the system after 500 s. The lines taking into account
trace metals (Met & No_Oz and Met & Oz)
are showing smaller concentration variations than those which
do not (No_Met & No_Oz and No_Met & Oz).
Moreover, the presence of ozone do not change anything if metal
is added (Met & Oz is with ozone, Met & No_Oz
is without), but become determinant is trace metals are neglected
(No_Met & Oz with and No_Met & No_Oz without
ozone).
Very small amounts of dissolved metals, 0.01 µM for Fe and 0.001 µM for Cu, decrease the radical concentration in presence of ozone but increase without ozone. That is the H2O2 importance against ozone in non polluted cloud drops is expanded. In presence of trace metals, the methanol decrease is close to a first order law, showing a straight line if "log[Methanol]" is expressed as function of time. This make computation easier.
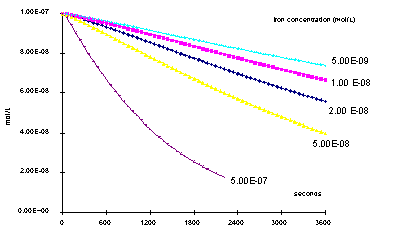
But the system becomes very sensitive to trace metals concentrations,
as shown by the figure left, plotting methanol concentration variations
versus time with various dissolved trace metal amounts, the ratio
to iron remaining constant.
Conclusion:
References
[1] Graedel T.E., Mandich M.L. and Weschler C.J. (1986), "Kinetic Model Studies of Atmospheric Droplet Chemistry: 2. Homogeneous Transition Metal Chemistry in Raindrops", J. Geophys. Res., 91 D4, 5205-5221.
[2] Jacob D.J., Gottlieb E.W. and Prather M.J. (1989), "Chemistry of a Polluted Cloudy Boundary Layer", J. Geophys. Res., 94 D10, 12975-13002.
[3] Lelieveld J. and Crutzen P.J. (1991), "The role of Clouds in Tropospheric Photochemistry", J. Atmos. Chem., 12, 229-267.
[4] Walcek C. J., Yuan H.H., and Stockwell W.R. (1997) "The influence of aqueous-phase chemical reactions on ozone formation in polluted and nonpolluted clouds", Atmos. Environ., 31, 1221-1237.
[5] Weschler C.J., Mandich M.L. and Graedel T.E. (1986), "Speciation, Photosensitivity, and Reactions of Transition Metal Ions in Atmospheric Droplets", J. Geophys. Res., 91 D4, 5189-5204.
[6]Willey J.D., Kiebert R.J. and Lancaster R.D. (1996), "Coastal Rainwater Hydrogen Peroxide: Concentration and deposition", J. Atmos. Chem., 25, 149-165.
[7] Losno R., (1989), "Chimie d'éléments minéraux en trace dans les pluis méditerranéenes", PhD Thesis, Université Paris 7, 215 pp.
[8] Losno R., Bergametti G., Carlier P. and Mouvier G. (1991), "Major ions in marine rainwater with attention to sources of alkaline and acidic species", Atmos. Environ., 25A, 763-770.
[9] Losno R., Colin J.L., Spokes L., Jickells T., Schulz M., Reberts A., Leermakers M., Meulemann C. and Bayens W. (1998), "Non-rain deposition significantly modifies rain samples at a coastal site", Atmos. Environ., 32, 3445-3455.
[10] Hoffmann P., Dedik A.N., Deutsch F., Sinner T., Weber S., Eichler R., Sterkel S., Sastri C.S. and Ortner H.M. (1997), "Solubility of single chemical compounds from an atmospheric aerosol in pure water, Atmos. Environ., 17, 2777-2785.
[11] Jickells T.D., Knap A.H. and Church T.M. (1984), "Trace Metals in Bermuda Rainwater", J. Geophys. Res., 89 D1, 1423-1428.
[12] Lim B., Losno R.,. Jickells T.D and Colin J.L. (1994), "The solubilities of aluminium, lead, copper and zinc in rain samples in the marine environment over the North Atlantic Ocean and the Mediteranean Sea." , Global Biogeochemical Cycles, 8, 349-362.
[13] Ross H.B. (1987), "Trace metals in precipitation in Sweden", Water Air and Soil Pollution, 36, 349-363.
[14] Spokes L.J., Jickells T.D. and Lim B. (1994),
"Solubilisation of aerosol trace metals by cloud processing:
a laboratory study", Geochim. Cosmochim. Acta, 58,
3281-3287.