L.I.S.A., Laboratoire Interuniversitaire des
Systèmes Atmosphériques (CNRS UMR 7583)
Faculté des Sciences, Universités Paris 7 et Paris
12, 61, av. du Gal de Gaulle
F94010 Créteil Cédex, FRANCE
e-mail: losno@lisa.univ-paris12.fr;
fax: (33 1) 45 17 15 64
Summary
Trace metals dissolved from aerosols are a key for aqueous atmospheric chemistry even in remote oceanic clouds. The aerosols weathering mechanisms show that H+ and OH are both implicated in the dissolution processes. An amorphous layer is created if the aerosol encountered several dissolution-drying cycles. Modelling the impact of trace metals in an oceanic cloud exhibit catalytic cycles involving dissolved Fe, Cu and H2O2. O3 has only a little influence on OH and HO2 generation and destruction.
Introduction
Several studies emphasised the potential role of transition metals in the formation of SO2, ozone and organic pollutants into the cloud droplets, especially Mn, Fe and Cu (Graedel et al., 1986; Jacob et al., 1989; Hoigné et al., 1994; Sedlak et al., 1997; Weschler et al., 1986; Erel et al., 1993; Matthijsen et al., 1995; Hoffmann & Jacob, 1984). Under conditions typically observed in atmospheric waters, transition metals like Fe, Cu and Mn can undergo a series of chemical and/or photochemical reactions that result in their rapid cycling between the oxidation states. The trace metals concentrations have a critical influence on the kinetic of the catalysed reactions (Losno, in press; Walcek et al., 1997; Berglund & Elding, 1995), and also on the global oxidation schemes in the atmosphere (Losno, in press).
The Influence of Trace Metals Leaching from Mineral Aerosols on Atmospheric Chemistry
Generally, in models, data measured in rainwater and on collected aerosols are used to integrate trace metals concentrations. These concentrations are considered to be identical with cloud waters ones. The rain physicochemical conditions, however, contrast with those in clouds (Jacob et al., 1985). Moreover, a cloud is a renewed system as opposed to precipitation. Thus, in view of the Cu(I) lifetime (1 s) (Losno, in press), it is strongly probable that concentrations of this metal are different in the two systems (Walcek et al., 1997). Therefore, in order to obtain a fair estimate of the contribution from this metal catalysis on the atmospheric chemistry, it is of particular importance to find a relation between measured and required data. Since the only source of trace metals in the aqueous phase is the solubilisation of aerosols, solid to soluble phase transfer mechanisms have to be studied.
Experiments of particle/water interaction were performed in an open flow reactor, using aerosol concentration, pH conditions and timescales representative of cloud water. This experimental system aims to simulate the water condensation on an aerosol particle likely to be encountered in clouds, and assess whether metal solubility is affected by pH. Aerosols used in this experiment are Saharan aerosol like material.
Major results
pH is one of the main control of the solubility of metals, in cloud waters and precipitation (Jickells et al., 1992; Spokes et al., 1994; Statham & Chester, 1988). For some trace metals, the relationship between pH and solubility is not, however, a simple one. Dissolution experiments have been conducted for typical pH of cloud and rain waters. To point out the effects of varying pH on dissolution, the advancement is an useful comparison parameter.

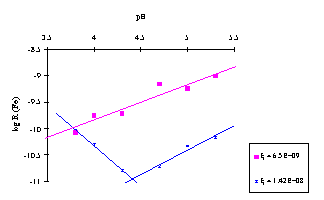
It appears that Fe dissolution rate at the beginning of the experiment (low ) increases regularly with pH (Fig. 1). Later (higher ), the iron dissolution rate reach a minimum at pH 4.3, suggesting two dissolution behaviours operate over longer periods of time; one at pH > 4.3, and a different one at pH < 4.3. The same trend can be observed for Cu with a minimum rate at pH 4.5. These observations suggest that both Cu and Fe are released by dissimilar forms at the beginning (low ) and the end (hig ) of the experiment. The form released at the beginning, probably an amorphous phase, seems to be completely removed after several minutes of dissolution. Moreover, the dissolution rate law can be expressed to sum both dissolution fractions: R = k[H+]a + k'[H+]-b. The slope indicating the reaction is controlled by H+ if negative, by OH if positive.
Similar compound rate laws describing mineral dissolution have yet been summarised (Stumm & Morgan, 1996). Typically, the proton-controlled mechanism dominates dissolution for pH < pHpzc and hydroxyl-controlled dissolution dominates overall dissolution for pH > pHpzc. The point of zero charge of mineral depends on pH, concentrations of all ions, hydration and crystallinity (Stumm & Morgan, 1996).
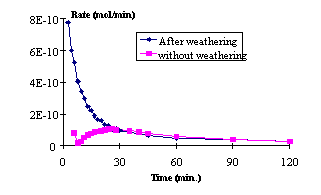
In order to clarify the probable impact of the surface crystallinity on trace metals solubilisation, two experiments were carried out. The one with original aerosol, and a second with weathered aerosol: these aerosols have already sustained a dissolution experiment so as to simulate a condensation/evaporation cycle. The results of these experiments (Fig. 2) show two different shapes according to the aerosol. For weathered aerosols, the dissolution rate is first very quick, as opposed to fresh particles, then the rate decrease up to relative low values. Accordingly, the solubilisation behaviour is different if aerosols have already sustained an evaporation. A probable amorphous phase is formed in surface, which is much more soluble than the initial crystalline phase.
From these results, a weathering mechanism can be proposed for original Saharan aerosol. First, the dissolution of the crystalline phase is activated by H+ and/or OH-: the most soluble elements therefore pass into the aqueous phase. However, a residual layer containing the less soluble elements (mainly SiO2) will form. Thus, for a continued efficient dissolution, H+, OH- and the released species must diffuse through this layer.
Two important points have been clarified to model the metal leaching influence on the atmospheric chemistry: Firstly, the pH influence on metals solubilisation has been pointed out and a relationship between pH and metal solubility is given. Secondly, the effect of the surface crystallinity has been emphasised. A possible solubilisation mechanism can be proposed in respect to these results.
Modelling trace metals acting as catalysts in non polluted clouds: multiphase chemistry in non polluted area.
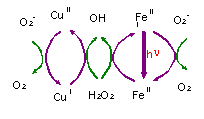
| Compound | Initial concentration | Reference |
| O3 | 3.0 E-10 mol.L1 | 25 ppb gaseous |
| H2O2 | 10 µmol.L1 | Willey et al.., 1996 |
| H+ | 10 µmol.L1 (pH = 5) | Losno et al., 1991 |
| Soluble Fe | 5.0 E8 mol.L1 | Hoffmann et al., 1997; Jickells et al., 1984 |
| Soluble Mn | 3.0 E9 mol.L1 | Hoffmann et al., 1997; Lim et al., 1994 |
| Soluble Cu | 1.0 E-9 mol.L1 | Lim et al., 1994; Spokes et al., 1994 |
Two coupled catalytic cycles drive all the reaction scheme into the cloud droplet: One with iron and other with copper. Gas exchange is fast enough to be considered as equilibrium, but metals exchange between particulate and dissolved phase are of major importance. The chemistry inside the cloud droplet in remote areas can be well described by around 40 reactions (Graedel et al., 1986; Jacob et al., 1989; Lelieved & Crutzen, 1991; Walcek et al, 1997; Weschler et al., 1986). But most of them are not important because not fast enough, and the system can be summarised with 7 equations including two catalytic cycles (Fig. 3). The initial concentrations are taken following next table:
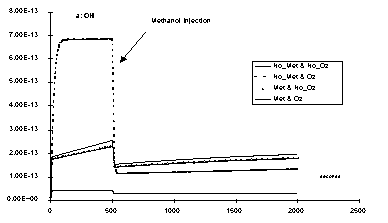
Fig. 4: OH radical concentrations (mol.L1) versus time if methanol (107 mol.L1) is added to the system after 500 s. The lines taking into account trace metals (Met & No_Oz and Met & Oz) are showing smaller concentration variations than those which do not (No_Met & No_Oz and No_Met & Oz). The presence of ozone do not change anything if metal is added (Met & Oz is with ozone, Met & No_Oz is without), but become determinant is trace metals are neglected (No_Met & Oz with and No_Met & No_Oz without ozone).
As the system reacts very fast to irradiation changes, a steady state is reached after less than 1 second for Cu, but take several minutes for Fe. Moreover, the catalytic cycles generate high flux for the production and the destruction of radicals, and so the steady state concentration of these radicals remain unchanged if amounts of other reactive species are added. Fig. 4 illustrates this by comparing a case were trace metals are present and a case were no trace metals are added. We have chosen methanol as a reducing species and ozone as an oxidising one. Very small amounts of dissolved metals decrease the radical concentration in presence of ozone but increase it without ozone. That is the H2O2 importance against ozone in non polluted cloud drops is expanded. But the system becomes very sensitive to trace metals concentrations.
Adding trace metals to the model completely changes the reaction scheme and makes the system very less sensitive to other reactive species. This can make computation more simple because added reactive species can be consider as 1st order law. One of the major result obtained here in this scope, is the ozone behaviour which act as a minor constituent with trace metals catalytic cycles.
References
Berglund J. and L. I. Elding; Manganese-catalysed autoxidation of dissolved sulfur dioxide in the atmospheric aqueous phase, Atmos. Environ. 29 (1995) 1379-1391.
Erel Y., S. O. Pehkonen and M. R. Hoffmann; redox Chemistry of Iron in Fog and Stratus Clouds, J. Geophys. Res. 98 (1993) 18,423-18,434.
Graedel T.E., M. L. Mandich and C. J. Weschler; Kinetic model studies of atmospheric droplet chemistry, 2. Homogeneous transition metal chemistry in raindrops., J. Geophys. Res. 91 D4 (1986) 5225-5221.
Hoffmann M.R. and D. J. Jacob; Kinetics and mechanism of the catalytic oxidation of dissolved sulfur dioxide in aqueous solution: an application to nighttime fog water chemistry, Boston 3, Butterworth, (1984), 101-172.
Hoffmann P., Dedik A.N., Deutsch F., Sinner T., Weber S., Eichler R., Sterkel S., Sastri C.S. and Ortner H.M.; Solubility of single chemical compounds from an atmospheric aerosol in pure water, Atmos. Environ. 17 (1997) 2777-2785.
Hoigné J., Y. Zuo and L. Nowell; Photochemical reactions in Atmospheric waters: Role of dissolved iron species, Boca Raton F.L. Lewis publishers, (1994).
Jacob D.J., J. M. Waldman, J. W. Munger and M. R. Hoffmann; Chemical composition of fogwater collected along the California coast, Environ. Sci. Technol. 19 (1985) 730-736.
Jacob D.J., E. W. Gottlieb and M. J. Prather; Chemistry of a polluted cloudy boundary layer, J. Geophys. Res. 94 (1989) 12,975-13,002.
Jickells T.D., Knap A.H. and Church T.M.; Trace Metals in Bermuda Rainwater, J. Geophys. Res., 89 D1 (1984) 1423-1428.
Jickells T.D. , T. D. Davies, M. Tranter, S. Landsberger, K. Jarvis and P. Abrahams; Trace elements in snow samples from the scottish highlands: Sources and dissolved/particulate distributions, Atmos. environ. 26 A (1992) 393-401.
Lelieveld J. and Crutzen P.J.; The role of Clouds in Tropospheric Photochemistry, J. Atmos. Chem. 12 (1991) 229-267.
Lim B., Losno R.,. Jickells T.D and Colin J.L.; The solubilities of aluminium, lead, copper and zinc in rain samples in the marine environment over the North Atlantic Ocean and the Mediteranean Sea, Global Biogeochemical Cycles 8 (1994) 349-362.
Losno R., Bergametti G., Carlier P. and Mouvier G.; Major ions in marine rainwater with attention to sources of alkaline and acidic species, Atmos. Environ., 25A, (1991) 763-770.
Losno R., Trace metals acting as catalysts in a marine cloud: a box model study, Physics and Chemistry of the Earth, in press.
Matthijsen J., P. J. H. Builtjes and D. L. Sedlak; Cloud model experiments of the effect of iron and copper on tropospheric ozone under marine and continental conditions, Met. Atmos. Phys. 57 (1995) 43-60.
Sedlak D.L., J. Hoigné, M. M. David, R. N. Colvile, E. Seyffer, K. Acker, W. Wiepercht, J. A. Lind and S. Fuzzi; The cloudwater chemistry of iron and copper at Great Dun Fell, UK., Atmos. Environ. 31 (1997) 2515-2526.
Spokes L.J. , T. D. Jickells and B. Lim; Solubilisation of aerosol trace metals by cloud procesing: a laboratory study., Geochim. Cosmochim. Acta 58 (1994) 3281-3287.
Statham P.J. and R. Chester; Dissolution of manganese from marine atmospheric particulates into seawater and rainwater, Geochim. Cosmochim. Acta 52 (1988) 2433-2437.
Stumm W. and J. J. Morgan; Aquatic Chemistry, New-York Wiley-Interscience, (1996), 1022 pp.
Walcek C.J., H. H. Yuan and W. R. Stockwell; The influence of aqueous-phase chemical reactions on ozone formation in polluted and nonpolluted clouds., Atmos. Environ. 31 (1997) 1221-1237.
Weschler C.J., M. L. Mandich and T. E. Graedel; Speciation, Photosensitivity, and reaction of transition metal ions in atmospheric droplets., J. Geophys. Res. 91 D4 (1986) 5189-5204.
Willey J.D., Kiebert R.J. and Lancaster R.D.; Coastal Rainwater Hydrogen Peroxide: Concentration and deposition, J. Atmos. Chem. 25 (1996) 149-165.